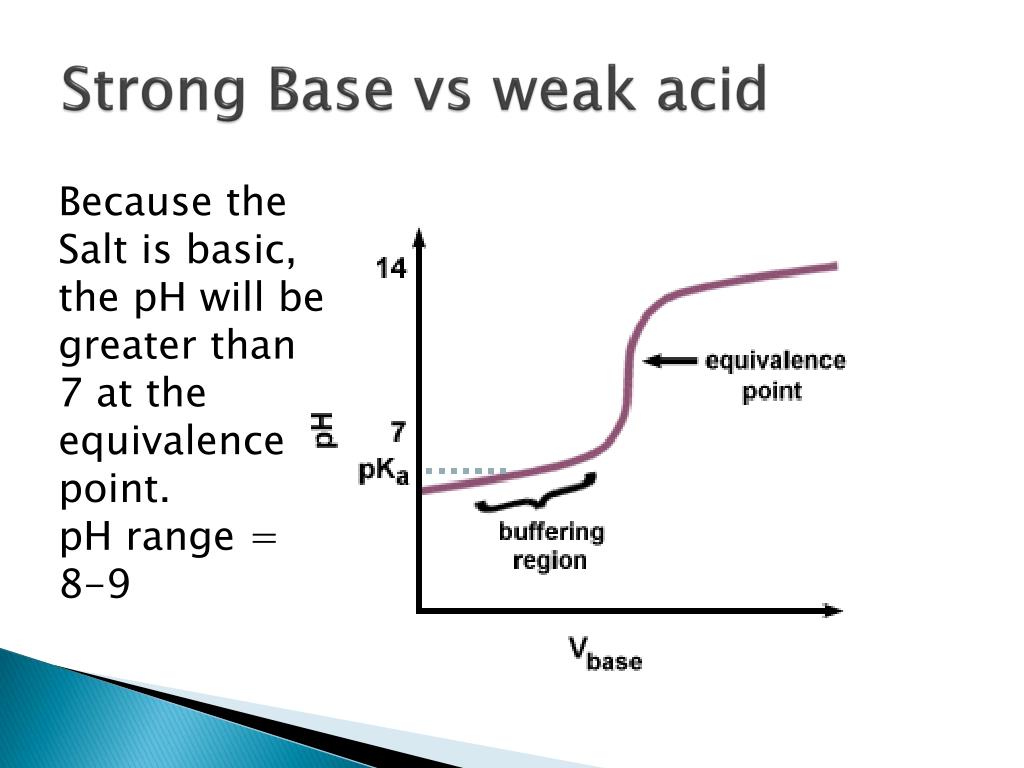
Indicators For Strong Acid And Strong Base . This page assumes that you know. titration of a strong acid with a strong base is the simplest of the four types of titrations as it involves a strong acid and strong base that. Selection of indicator in acid base titration. the graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of. The next diagram shows the ph curve for adding a strong acid to a strong base. Phenolphthalein is usually preferred because of its more easily seen colour change. ph colour indicators are molecules that: strong acid vs. A) are a weak acid or base (so they react with hydronium) b) have conjugate acid and.
from www.slideserve.com
A) are a weak acid or base (so they react with hydronium) b) have conjugate acid and. the graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of. This page assumes that you know. Selection of indicator in acid base titration. strong acid vs. Phenolphthalein is usually preferred because of its more easily seen colour change. ph colour indicators are molecules that: titration of a strong acid with a strong base is the simplest of the four types of titrations as it involves a strong acid and strong base that. The next diagram shows the ph curve for adding a strong acid to a strong base.
PPT Acid Base Titrations PowerPoint Presentation, free download ID
Indicators For Strong Acid And Strong Base Phenolphthalein is usually preferred because of its more easily seen colour change. Phenolphthalein is usually preferred because of its more easily seen colour change. the graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of. Selection of indicator in acid base titration. ph colour indicators are molecules that: This page assumes that you know. strong acid vs. The next diagram shows the ph curve for adding a strong acid to a strong base. A) are a weak acid or base (so they react with hydronium) b) have conjugate acid and. titration of a strong acid with a strong base is the simplest of the four types of titrations as it involves a strong acid and strong base that.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Indicators For Strong Acid And Strong Base Phenolphthalein is usually preferred because of its more easily seen colour change. Selection of indicator in acid base titration. titration of a strong acid with a strong base is the simplest of the four types of titrations as it involves a strong acid and strong base that. the graph shows the results obtained using two indicators (methyl red. Indicators For Strong Acid And Strong Base.
From www.slideserve.com
PPT Properties of Acids PowerPoint Presentation, free download ID Indicators For Strong Acid And Strong Base titration of a strong acid with a strong base is the simplest of the four types of titrations as it involves a strong acid and strong base that. ph colour indicators are molecules that: strong acid vs. the graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of. Phenolphthalein is. Indicators For Strong Acid And Strong Base.
From www.teachoo.com
Strength of Acids and Bases (How to find it?) Chemistry Teachoo Indicators For Strong Acid And Strong Base titration of a strong acid with a strong base is the simplest of the four types of titrations as it involves a strong acid and strong base that. Selection of indicator in acid base titration. the graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of. strong acid vs. The next. Indicators For Strong Acid And Strong Base.
From www.youtube.com
To study the pH change in the titration of strong base and strong acid Indicators For Strong Acid And Strong Base The next diagram shows the ph curve for adding a strong acid to a strong base. This page assumes that you know. ph colour indicators are molecules that: titration of a strong acid with a strong base is the simplest of the four types of titrations as it involves a strong acid and strong base that. the. Indicators For Strong Acid And Strong Base.
From www.pinterest.com.mx
Strong Acids and Bases MCAT Chemistry Cheat Sheet Study Guide StudyPK Indicators For Strong Acid And Strong Base The next diagram shows the ph curve for adding a strong acid to a strong base. the graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of. titration of a strong acid with a strong base is the simplest of the four types of titrations as it involves a strong acid and. Indicators For Strong Acid And Strong Base.
From www.youtube.com
acids,bases and salts indicators class10 by poonam malhotra YouTube Indicators For Strong Acid And Strong Base strong acid vs. ph colour indicators are molecules that: Phenolphthalein is usually preferred because of its more easily seen colour change. A) are a weak acid or base (so they react with hydronium) b) have conjugate acid and. Selection of indicator in acid base titration. This page assumes that you know. The next diagram shows the ph curve. Indicators For Strong Acid And Strong Base.
From www.teachoo.com
Strength of Acids and Bases (How to find it?) Chemistry Teachoo Indicators For Strong Acid And Strong Base titration of a strong acid with a strong base is the simplest of the four types of titrations as it involves a strong acid and strong base that. ph colour indicators are molecules that: The next diagram shows the ph curve for adding a strong acid to a strong base. the graph shows the results obtained using. Indicators For Strong Acid And Strong Base.
From www.slideshare.net
Acid base titration Indicators For Strong Acid And Strong Base ph colour indicators are molecules that: the graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of. strong acid vs. titration of a strong acid with a strong base is the simplest of the four types of titrations as it involves a strong acid and strong base that. A) are. Indicators For Strong Acid And Strong Base.
From www.slideserve.com
PPT Acids and Bases PowerPoint Presentation, free download ID6973259 Indicators For Strong Acid And Strong Base This page assumes that you know. Phenolphthalein is usually preferred because of its more easily seen colour change. A) are a weak acid or base (so they react with hydronium) b) have conjugate acid and. strong acid vs. Selection of indicator in acid base titration. ph colour indicators are molecules that: the graph shows the results obtained. Indicators For Strong Acid And Strong Base.
From www.slideserve.com
PPT Acids and Bases PowerPoint Presentation, free download ID6776086 Indicators For Strong Acid And Strong Base the graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of. ph colour indicators are molecules that: This page assumes that you know. Phenolphthalein is usually preferred because of its more easily seen colour change. titration of a strong acid with a strong base is the simplest of the four types. Indicators For Strong Acid And Strong Base.
From www.teachoo.com
Acid Base Indicators All types [List with Examples] Teachoo Indicators For Strong Acid And Strong Base titration of a strong acid with a strong base is the simplest of the four types of titrations as it involves a strong acid and strong base that. the graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of. strong acid vs. ph colour indicators are molecules that: Selection of. Indicators For Strong Acid And Strong Base.
From www.slideserve.com
PPT AcidBase Titrations PowerPoint Presentation, free download ID Indicators For Strong Acid And Strong Base Phenolphthalein is usually preferred because of its more easily seen colour change. This page assumes that you know. titration of a strong acid with a strong base is the simplest of the four types of titrations as it involves a strong acid and strong base that. Selection of indicator in acid base titration. A) are a weak acid or. Indicators For Strong Acid And Strong Base.
From general.chemistrysteps.com
Strong AcidStrong Base Titrations Chemistry Steps Indicators For Strong Acid And Strong Base A) are a weak acid or base (so they react with hydronium) b) have conjugate acid and. strong acid vs. the graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of. titration of a strong acid with a strong base is the simplest of the four types of titrations as it. Indicators For Strong Acid And Strong Base.
From mmerevise.co.uk
pH Curves Questions and Revision MME Indicators For Strong Acid And Strong Base the graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of. This page assumes that you know. Selection of indicator in acid base titration. Phenolphthalein is usually preferred because of its more easily seen colour change. A) are a weak acid or base (so they react with hydronium) b) have conjugate acid and.. Indicators For Strong Acid And Strong Base.
From saylordotorg.github.io
AcidBase Titrations Indicators For Strong Acid And Strong Base strong acid vs. A) are a weak acid or base (so they react with hydronium) b) have conjugate acid and. The next diagram shows the ph curve for adding a strong acid to a strong base. Phenolphthalein is usually preferred because of its more easily seen colour change. the graph shows the results obtained using two indicators (methyl. Indicators For Strong Acid And Strong Base.
From artofsmart.com.au
Guide to HSC Chemistry Module 6 Acid/Base Reactions Indicators For Strong Acid And Strong Base the graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of. strong acid vs. Phenolphthalein is usually preferred because of its more easily seen colour change. Selection of indicator in acid base titration. A) are a weak acid or base (so they react with hydronium) b) have conjugate acid and. The next. Indicators For Strong Acid And Strong Base.
From byjus.com
Acid Base Titration Titration Curves, Equivalence Point & Indicators Indicators For Strong Acid And Strong Base strong acid vs. The next diagram shows the ph curve for adding a strong acid to a strong base. titration of a strong acid with a strong base is the simplest of the four types of titrations as it involves a strong acid and strong base that. This page assumes that you know. Phenolphthalein is usually preferred because. Indicators For Strong Acid And Strong Base.
From www.slideserve.com
PPT Acid Base Titrations PowerPoint Presentation, free download ID Indicators For Strong Acid And Strong Base ph colour indicators are molecules that: strong acid vs. The next diagram shows the ph curve for adding a strong acid to a strong base. titration of a strong acid with a strong base is the simplest of the four types of titrations as it involves a strong acid and strong base that. Phenolphthalein is usually preferred. Indicators For Strong Acid And Strong Base.